CBS News
FDA approves new Alzheimer’s treatment, donanemab from Eli Lilly

The Food and Drug Administration approved a new Alzheimer’s treatment called donanemab on Tuesday, clearing the way for the third addition to a new class of drugs aimed at slowing the brain’s decline in patients facing the early stages of the disease.
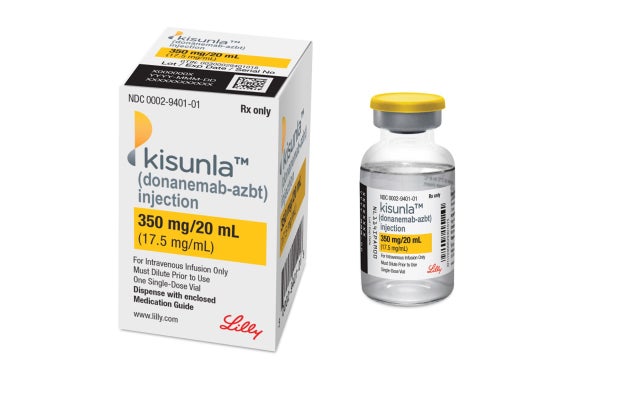
Branded as Kisunla by drugmaker Eli Lilly, donanemab’s approval follows years of setbacks and delays in getting the experimental Alzheimer’s treatment to market, despite promising clinical trial results.
Eli Lilly says the drug will be available within weeks following the approval.
The FDA previously rebuffed Eli Lilly’s request for accelerated approval last year, citing concerns about its long-term safety data. After Eli Lilly submitted more data to the FDA, the company said it expected the agency would decide on approval by the end of March.
That decision was delayed after the FDA scheduled an advisory committee to wrestle with questions over the drug’s safety issues and how effectiveness was measured in its trials. The panel ultimately voted unanimously last month in favor of the drug’s benefits outweighing its risks, for patients in the early stages of Alzheimer’s disease.
How does donanemab work?
Donanemab is part of a class of Alzheimer’s treatments called anti-amyloid monoclonal antibodies, which work to combat the buildup of a protein in the brain called amyloid plaque that has been linked to Alzheimer’s disease.
The antibody in donanemab targets amyloid plaques that have built up in patients by binding to and removing them from the brain.
Patients in Eli Lilly’s trials were given intravenous donanemab infusions for around half an hour, every four weeks. Depending on brain scans measuring amyloid levels in the brain, patients were able to stop taking the drug after as early as six months.
In its trials, the company says almost half of patients were able to meaningfully clear out amyloid after around a year after taking the drug. Patients saw no “rebound of amyloid plaque” in the year after treatment wrapped up.
Eli Lily
The only other Alzheimer’s treatment that works in a similar way on the market is lecanemab, branded as Leqembi by drugmakers Eisai and Biogen. An earlier drug called aducanumab (marketed as Aduhelm) from Biogen was discontinued in January.
Beyond effectiveness, Eli Lilly has also touted a handful of other reasons that patients might choose their drug instead of lecanemab.
Donanemab infusions are shorter and less frequent. Trial participants were also able to stop using the drug after amyloid plaque was removed, “which can result in lower treatment costs and fewer infusions,” a company spokesperson said.
How much will the treatment cost?
Eli Lilly says it will launch with a list price of $32,000 for 12 months of treatment, though the actual cost will depend on how long patients take the drug. Last year, Eisai defended its list price of $26,500 per year when it launched sales of Leqembi.
But most patients also do not pay the full list price for prescription drugs. For patients with Medicare Part B, the Centers for Medicare and Medicaid Services said donanemab will be covered in the same way it covers lecanemab (Leqembi), with patients paying a 20% coinsurance after they meet their deductible. These patients will need to get the drug from doctors enrolled in a study gathering data tracking its effectiveness.
“CMS is committed to helping people get timely access to treatments and improving care for people with Alzheimer’s disease and their families,” a CMS spokesperson said.
Eli Lilly noted in a statement: “The potential to complete treatment after a limited-duration course of therapy, along with 30-minute infusions once per month, could result in lower patient out-of-pocket treatment costs and fewer infusions compared to other amyloid-targeting therapies.”
How effective was the treatment for Alzheimer’s symptoms?
Eli Lilly measured donanemab’s effectiveness primarily through rating scales designed to measure the cognitive and functional decline caused by dementia symptoms in patients with early stages of Alzhiemer’s.
Compared with patients who received only a placebo, Eli Lilly said those who got the drug saw their decline slow by up to 22% at 76 weeks after first starting the donanemab infusions.
“Importantly, the magnitude of impact on these clinical endpoints meets, and in several respects exceeds prior approvals for demonstration of clinical benefit and effectiveness,” the company said of the results in a briefing document given to the FDA panel.
The company says this translated to effectively prolonging how long it took until patients stepped down into the next stage of Alzheimer’s disease.
What are the side effects of donanemab?
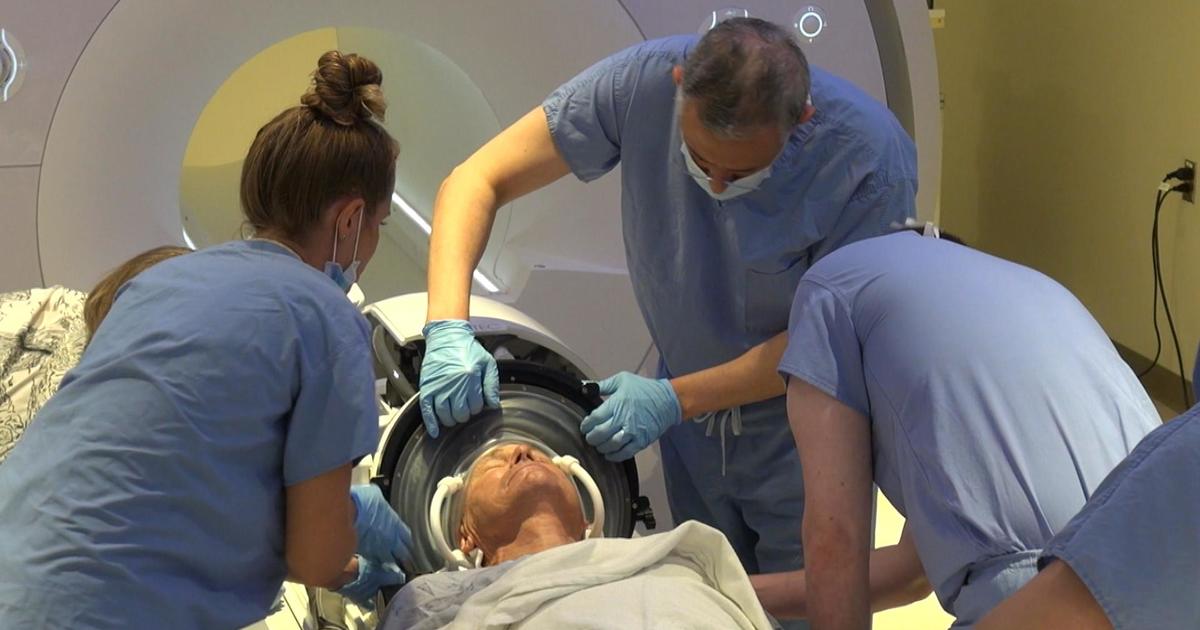
The labels for all of the anti-amyloid treatments greenlighted by the FDA to date for Alzheimer’s already carry a boxed warning about “amyloid-related imaging abnormalities” that can show up on MRI scans.
While these abnormalities generally result in no symptoms, they have been linked to rare but serious issues in some patients like brain function issues and seizures.
These abnormalities were seen in around a quarter of participants in Eli Lilly’s trials of donanemab. At least five deaths were reported in donanemab recipients in patients with these kinds of abnormalities, mostly from hemorrhages in the brain.
Eli Lilly says their trials of donanemab tested the drug in harder to treat patients than other treatments studied around the same time. That means the trial included older trial participants as well as those with a gene called APOE ε4 that can increase the risk of Alzheimer’s as well as these abnormalities.
Close to 1 in 10 trial participants who took donanemab also experienced a reaction to the infusion, compared to 0.5% of placebo participants. The most common symptoms included chills, skin reddening, nausea, shortness of breath, headache and chest pain.
Approximately 3% of donanemab-treated participants developed hypersensitivity to the infusion, including 0.3% who had a severe allergic reaction.
CBS News
3 Columbia University administrators ousted from posts over controversial texts

NEW YORK – Three administrators have been “permanently removed from their positions” at Columbia College and “remain on leave” over texts they exchanged during an on-campus event about Jewish life at the school, Columbia University’s president announced Monday.
It happened during the school’s reunion weekend at the end of May. The program was called “Jewish Life on Campus: Past, Present and Future,” and took place a month after university leaders called in police to clear pro-Palestinian protesters out of an occupied administration building and dismantle a tent encampment that had threatened to disrupt graduation ceremonies.
One of the controversial messages suggested a panelist could have used recent campus protests as a fundraising opportunity. Another appeared critical of a campus rabbi’s essay about antisemitism.
Texts “touched on ancient antisemitic tropes”
“This incident revealed behavior and sentiments that were not only unprofessional, but also, disturbingly touched on ancient antisemitic tropes,” Columbia President Minouche Shafik wrote in a letter to the Columbia community. “Whether intended as such or not, these sentiments are unacceptable and deeply upsetting, conveying a lack of seriousness about the concerns and the experiences of members of our Jewish community that is antithetical to our University’s values and the standards we must uphold in our community.”
Shafik said the school will “launch a vigorous program of antisemitism and antidiscrimination training for faculty and staff this fall.” Similar training will also be given to students.
Columbia Provost Angela Olinto wrote that the administrators’ conduct was “wrong and contrary to the mission and values of our institution. It revealed, at best, an ignorance of the history of antisemitism.”
Columbia College Dean Josef Sorett, whose text messages were among those published by the Free Beacon, will continue to lead the college after apologizing and committing to working to fix damage caused by the text exchanges, Olinto said. He and his administration will be expected to “deliver concrete change in combating antisemitism and discrimination and creating a fully inclusive environment,” Olinto wrote.
“While not intended as such, some of the text messages exchanged may call to mind antisemitic tropes,” Sorett said in a letter Monday to the Columbia College community. “Any language that demeans members of our community, or divides us from one another, is simply unacceptable.”
“I am deeply sorry that this happened in a community that I lead- and, that I was part of any of the exchanges, and I pledge to spearhead the change we need to ensure this never happens again,” Sorett continued. He said “the loss of trust and the pain this incident has caused, particularly to the Jewish members of our community, must be fully repaired.”
The university did not identify the administrators, who were initially put on leave in June after images of their text exchange were published online by the Washington Free Beacon, a conservative news outlet. The content of the texts was additionally released by the House Committee on Education and the Workforce last week.
CBS News
7/8: CBS News 24/7 Episode 2

Watch CBS News
Be the first to know
Get browser notifications for breaking news, live events, and exclusive reporting.
CBS News
New cyberattack targets iPhone Apple IDs. Here’s how to protect your data.

A new cyberattack is targeting iPhone users, with criminals attempting to obtain individuals’ Apple IDs in a “phishing” campaign, security software company Symantec said in an alert Monday.
Cyber criminals are sending text messages to iPhone users in the U.S. that appear to be from Apple, but are in fact an attempt at stealing victims’ personal credentials.
“Phishing actors continue to target Apple IDs due to their widespread use, which offers access to a vast pool of potential victims,” Symantec said. “These credentials are highly valued, providing control over devices, access to personal and financial information, and potential revenue through unauthorized purchases.”
Consumers are also more likely to trust communications that appear to come from a trusted brand like Apple, warned Symantec, which is owned by Broadcom, a maker of semiconductors and infrastructure software.
The malicious SMS messages appear to come from Apple and encourage recipients to click a link and sign in to their iCloud accounts. For example, a phishing text could say: “Apple important request iCloud: Visit signin[.]authen-connexion[.]info/icloud to continue using your services.” Recipients are also asked to complete a CAPTCHA challenge in order to appear legitimate, before they’re directed to a fake iCloud login page.
Such cyberattacks are commonly referred to as “smishing” schemes in which criminals use fake text messages from purportedly reputable organizations, rather than email, to lure people into sharing personal information, such as account passwords and credit card data.
How to protect yourself
Be cautious about opening any text messages that appear to be sent from Apple. Always check the source of the message — if it’s from a random phone number, the iPhone maker is almost certainly not likely not to be the sender. iPhone users should also avoid clicking on links inviting people to access their iCloud account; instead, go to login pages directly.
Apple urges users to always enable two-factor authentication for Apple ID for extra security and to make it harder to access to your account from another device. It is “designed to make sure that you’re the only person who can access your account,” Apple said.
The Federal Trade Commission also recommends setting up your computer and mobile phone so that security software is updated automatically.