CBS News
Counterfeit weight loss drugs sold online, feeding demand for cheaper options

Slickly designed websites. Unbelievable markdowns. A simple online search for semaglutide, the active ingredient in Ozempic or Wegovy, can lead consumers down a rabbit hole of seemingly reputable online pharmacies in search of weight loss drugs at a discounted price. The temptation is clear for many Americans, but some of those deals come with a potentially deadly cost.
U.S. consumers pay higher prices for weight loss drugs than any other country in the world, according to a 2023 KFF study. Cheaper alternatives online, however, are not always what they seem.
“People are really taking a risk by ordering these products online and not knowing the supplier that’s sending it to them,” said Salvatore Ingrassia, port director for Customs and Border Protection at New York’s JFK Airport.
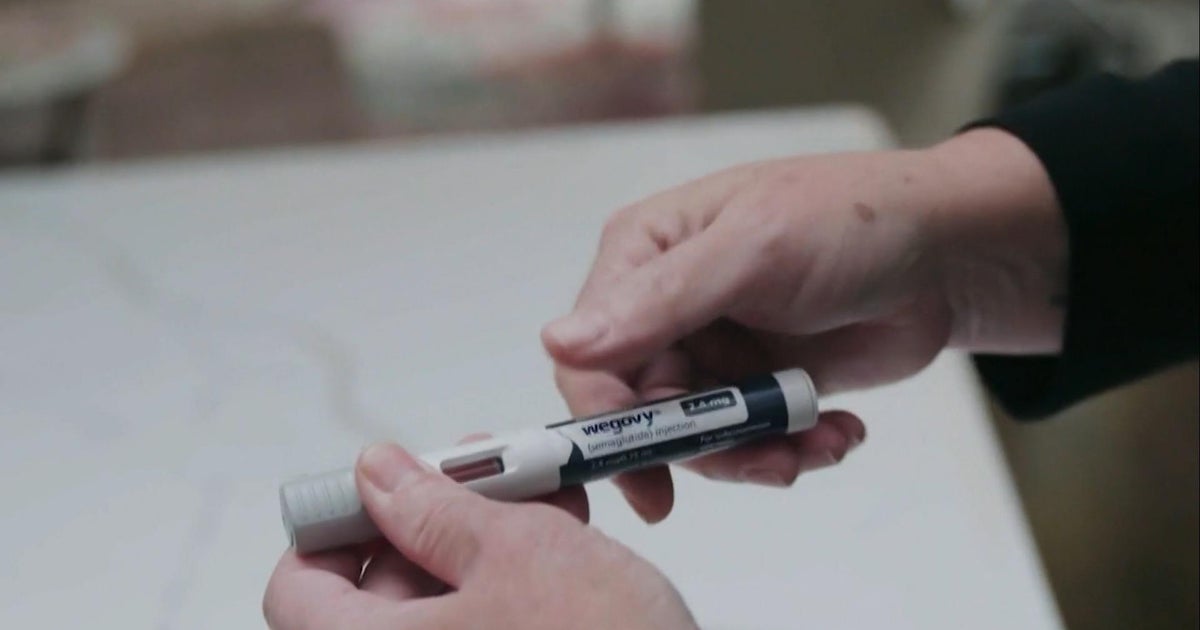
CBP agents are on the front lines, intercepting counterfeit prescription drugs, which pour into the country. These include knockoffs of popular weight loss drugs.
Customs officials allowed CBS News a rare look into their operations, showcasing seized misbranded or counterfeit medications. Over two days, agents confiscated products from countries including Israel and Bangladesh. One shipment of suspected counterfeit Wegovy was hidden inside a Mickey Mouse puzzle for children.
While these counterfeit drugs may look legitimate, they often contain dangerous substances.
CBS News
“We’ve found things like antifreeze and incorrect amounts of active ingredients in these products,” said Ingrassia.
In September, customs agents in Cincinnati’s port inspected 63 shipments containing apparently counterfeit and unapproved medications. That included several boxes containing counterfeit Ozempic, Wegovy and Trulicity injections. Other medications, including Botox and minoxidil, used for hair growth, were also seized by the authorities.
The FDA is taking action
The Food and Drug Administration has issued warnings to multiple online pharmacies selling suspected counterfeit products. The letters demand the sites stop selling the products that are in violation of FDA’s standards or face legal action. Despite their efforts, a quick internet search by CBS News in September 2024 found these websites remain active or redirect users to other sites offering these questionable drugs.
The Food and Drug Administration told CBS News it cannot comment on enforcement actions.
The health concerns of counterfeits
The dangers to health are real. Novo Nordisk reported one counterfeit pen contained the diabetes medicine insulin. The user was hospitalized after administering the counterfeit pen.
Authorities have arrested individuals involved in selling counterfeit drugs, including a Long Island woman suspected of selling misbranded Ozempic online. The woman pleaded not guilty to the charges and is out on bond. Court documents show that one victim reported lesions and infections after using the medication.
Former FDA director of investigations in the Chicago area, Ricki Chase, an expert in tracking counterfeit products, warns consumers to stay vigilant.
“If you’re buying a drug legitimately priced at thousands of dollars for just $65, you should be asking questions,” she said.
What can consumers do?
Chase advises consumers to only buy these drugs with a prescription from well-known pharmacies. Real weight-loss medication pens should have unique identification codes and proper labeling.
If the label smears or appears misaligned, it’s likely counterfeit. Uneven glue seams, lot numbers with crooked print, and cheap looking colors are also signs the products could be counterfeit, according to Chase.
Ultimately, she believes the best defense is using sound judgment.
“Don’t risk your health trying to get healthy,” she said.
Novo Nordisk, the maker of Ozempic, Rybelsus and Wegovy, said in a statement to CBS News: “It’s important that patients are aware that Novo Nordisk is the only company in the U.S. with FDA-approved medicines containing the main ingredient semaglutide. FDA has not approved any generic versions of semaglutide.”
Eli Lilly, makers of Mounjaro and Zepbound, also used for weight loss, told CBS News: “Lilly has taken steps to help inform people about the risks posed by counterfeit, fake, and unsafe or untested products, creating lilly.com/real-medicine which includes a tool to help determine whether you have genuine Lilly product. “
The National Association of Boards of Pharmacy has sent up a Safe Site Search Tool which allows users to verify if a website selling medications has been vetted. You can also review a list of the current websites that have been accredited by the NABP here.
CBS News
How to watch the Minnesota Vikings vs. Chicago Bears NFL game today: Livestream options, more

Getty Images
The Minnesota Vikings will take on the Chicago Bears today. The Vikings are currently 8-2, an impressive run so far this season, and will be looking to add a fourth win to their current streak after last Sunday’s 23-13 win against the Tennessee Titans. The Bears, on the other hand, are entering this game on the heels of a four-game losing streak after a tough 20-19 loss against the Green Bay Packers last Sunday.
Here’s how and when you can watch the Vikings vs. Bears game today, whether or not you have cable.
How and when to watch the Minnesota Vikings vs. Chicago Bears
The Vikings vs. Bears game will be played on Sunday, November 24, 2024 at 1:00 p.m. ET (11:00 a.m. PT). The game will air on Fox and stream on Fubo and the platforms featured below.
How and when to watch the Minnesota Vikings vs. Chicago Bears game without cable
You can watch this week’s NFL game on Fox via several streaming services. All you need is an internet connection and one of the top options outlined below.
Fubo offers you an easy, user-friendly way to watch NFL games on CBS, Fox, NBC, ABC, ESPN, and NFL Network, plus NCAA football channels. The Pro tier includes 200+ channels and unlimited DVR, while the Elite with Sports Plus tier adds NFL RedZone and 4K resolution. New subscribers get a seven-day free trial and all plans allow streaming on up to 10 screens simultaneously.
You can watch today’s game with a subscription to Sling’s Orange + Blue tier, which includes ESPN, ABC, NBC, and Fox. The plan offers 46 channels with local NFL games, nationally broadcast games and 50 hours of DVR storage. For complete NFL coverage, add Paramount+ to get CBS games, or upgrade with the Sports Extra add-on for additional sports channels like Golf Channel, NBA TV and NFL RedZone.
Watching NFL games, including Fox broadcasts, is simple with Hulu + Live TV, which includes 90 channels, unlimited DVR storage, and access to NFL preseason games, live regular season games and studio shows. The service includes ESPN+ and Disney+ in the subscription.
Want to watch today’s game live on your smartphone? If so, NFL+ streaming service is the solution you’re looking for. It lets you watch NFL Network and out-of-market games on mobile devices, with an upgrade option to NFL+ Premium that includes NFL RedZone for watching up to eight games simultaneously. Note that NFL+ only works on phones and tablets, not TVs.
CBS News
How to watch the Detroit Lions vs. Indianapolis Colts NFL game today: Livestream options, more

Nic Antaya/Getty Images
The Detroit Lions will face off against the Indianapolis Colts today. The Lions enter this game as top contenders with a near-perfect record of 9-1 so far this season. The Colts, who are 5-6 this season, could have a tough game on their hands against the Lions but will be looking to rack up another win after prevailing over the New York Jets in a tight game last Sunday.
Here’s how and when you can watch the Colts vs. Lions game today, whether or not you have cable.
Here’s how and when to watch the Detroit Lions vs. Indianapolis Colts
The Lions vs. Colts game will be played on Sunday, November 24, 2024 at 1:00 p.m. ET (11:00 a.m. PT). The game will air on Fox and stream on Fubo and the platforms featured below.
How and when to watch the Detroit Lions vs. Indianapolis Colts game without cable
You can watch this week’s NFL game on Fox via several streaming services. All you need is an internet connection and one of the top options outlined below.
Experience NFL action like never before with Fubo’s comprehensive sports streaming platform. From Sunday showdowns to primetime matchups, catch every NFL game across major networks including CBS, Fox, NBC, ABC, ESPN and NFL Network. Choose the Pro package to unlock 200+ channels and limitless DVR storage, or elevate your game-day experience with the Elite with Sports Plus package, featuring NFL RedZone’s commercial-free scoring highlights and stunning 4K quality.
Test drive the service with a no-commitment seven-day free trial, and share the excitement with family and friends — Fubo supports simultaneous streaming on up to 10 devices, so everyone can watch their favorite teams.
You can watch today’s game with a subscription to Sling’s Orange + Blue tier, which includes ESPN, ABC, NBC, and Fox. The plan offers 46 channels with local NFL games, nationally broadcast games, and 50 hours of DVR storage. For complete NFL coverage, add Paramount+ to get CBS games, or upgrade with the Sports Extra add-on for additional sports channels like Golf Channel, NBA TV and NFL RedZone.
Watching NFL games, including Fox broadcasts, is simple with Hulu + Live TV, which includes 90 channels, unlimited DVR storage, and access to NFL preseason games, live regular season games and studio shows. The service includes ESPN+ and Disney+ in the subscription.
Want to watch today’s game live on your smartphone? If so, NFL+ streaming service is the solution you’re looking for. It lets you watch NFL Network and out-of-market games on mobile devices, with an upgrade option to NFL+ Premium that includes NFL RedZone for watching up to eight games simultaneously. Note that NFL+ only works on phones and tablets, not TVs.
CBS News
How to watch the New England Patriots vs. Miami Dolphins NFL game today: Livestream options, more

Getty Images
The New England Patriots will face off against the Miami Dolphins in a game today at the Hard Rock Stadium in Miami. The Patriots have had an uneven season so far, coming into the game with a record of 3-8, including a 28-22 loss to the Los Angeles Rams on November 17. The Dolphins, however, haven’t fared much better this season as they enter the game with a record of 4-6, although they are coming off two wins in a row, the latest against the Las Vegas Raiders last weekend.
Keep reading to find out how and when to watch the New England Patriots vs. Miami Dolphins game today, even without cable.
CBS, Paramount+ and CBS Essentials are all subsidiaries of Paramount Global.
How and when to watch the New England Patriots vs. Miami Dolphins game today
The New England Patriots vs. Miami Dolphins game will be played on Sunday, November 24, 2024 at 1:00 p.m. ET (10:00 a.m. PT). The football game will be shown on CBS and streamed on Paramount+ and the platforms noted below.
How and when to watch the New England Patriots vs. Miami Dolphins game without cable
While CBS is available with many basic cable packages, you’ll have other viewing options, too for the Patriots-Dolphins game. Just understand that the below streaming options will require the use of an internet provider:
Paramount+: Watch CBS-aired NFL games without cable
With Paramount+ you’ll have multiple viewing options to choose from. You can catch NFL games on the Paramount+ Essential tier for just $7.99 each month or you can watch college football with a Paramount+ with Showtime subscription for $12.99 monthly. In addition to live streams of NFL games airing on CBS, you’ll get to watch additional live sporting events including NCAA college football, PGA Tour golf, soccer and more.
Get started with Paramount+ here today.
Amazon Prime Video: Add Paramount+ to your existing subscription
Already have an Amazon Prime Video account? Simply add Paramount+ to your current subscription to watch all the CBS-aired NFL games in addition to Paramount+ originals. The same prices from above apply, depending on which tier you choose. Not sure which is best for you? Don’t worry. Both options come with a free seven-day trial that can help you decide.
Watch the Patriots-Dolphins game on Amazon Prime Video.
Fubo: Watch the Patriots-Dolphins game for free
Looking for an inexpensive way to watch football? Fubo could be the best way to do so. The live TV streamer is currently offering a seven-day free trial and $30 off of your first month’s subscription. Once subscribed, you’ll gain access to all of their live sporting events immediately. And there will be a lot to choose from. Not only does Fubo come with access to NFL games airing on your local CBS channel, it also includes Fox Sunday NFC games, “Sunday Night Football” on NBC, “Monday Night Football” on ABC and ESPN and all of the games that air on the NFL Network. So don’t wait.
Get started with Fubo online now.