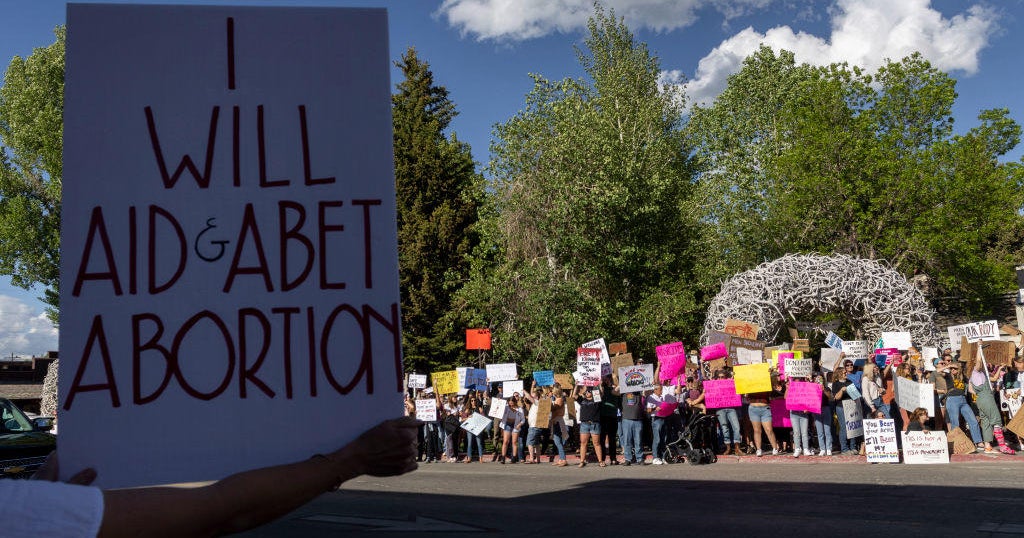CBS News
Anti-abortion rights groups say they can reverse the abortion pill. That’s fraud, some states say.

Some anti-abortion rights groups are selling a procedure called “abortion pill reversal,” which they claim can help women halt medical abortions. That claim is now being challenged by state officials who allege the groups are veering into false advertising and fraud.
Heartbeat International, an anti-abortion rights group, and 11 other anti-abortion organizations were sued Monday by New York Attorney General Letitia James, who alleged the organizations are making “false and misleading statements to advertise an unproven treatment.” The complaint follows a similar lawsuit filed by California Attorney General Rob Bonta in September.
Heartbeat has countersued, asking the courts to throw out California’s lawsuit on the grounds that it infringes its First Amendment rights. It also asserts that abortion pill reversal is safe and effective.
The litigation comes at a time when the so-called abortion pill — actually a combination of two drugs — accounts for almost two-thirds of abortions in the U.S., according to the Guttmacher Institute, which researches reproductive rights. More women are turning to medication abortion, rather than surgery, partly because it allows them to seek care privately and manage the process at home.
Meanwhile, abortion access has faced growing restrictions across much of the U.S. following the Supreme Court’s 2022 decision overturning Roe v. Wade. The high court is also currently considering a case that could ultimately curtail use of the abortion pill across the U.S.
In taking aim at the anti-abortion groups, New York and California are turning to state laws that prohibit deceptive business practices as well as false advertising — in effect, both are asking courts to rule on whether the clinics’ advertisements for abortion pill reversal treatment amount to fraud.
New York and California also want the courts to block Heartbeat and affiliated anti-abortion clinics from continuing to promote abortion pill reversal because they are allegedly misleading consumers.
For its part, Heartbeat International told CBS MoneyWatch that James’ lawsuit is a “clear attempt to censor speech.”
“By singling out these organizations solely because they offer an alternative to abortion, she is not only violating their rights but also denying women access to care and support as they seek to try and continue their pregnancies,” Heartbeat said in an email.
What is “abortion pill reversal”?
Medication abortion involves taking two pills: mifepristone, which was approved by the Food and Drug Administration in 2000, and misoprostol. To begin the process, a woman first takes mifepristone, which stops the pregnancy from growing. (The Supreme Court’s case involving the abortion pill is focused on mifepristone.)
About 24 to 48 hours after that, the woman takes misoprostol, which causes the uterus to contract and abort the fetus.
As this process has become the most common abortion treatment, anti-abortion clinics have turned to promoting a technique they claim can “reverse” the effect of the abortion pill. But that claim is misleading because it implies the treatment can undo an abortion, James alleges.
The anti-abortion clinics “imply the impossible — that fetal tissue that has been expelled from the uterus due to a completed abortion can be returned to the uterus. It cannot,” the New York lawsuit alleges.
In reality, the treatment is aimed at halting the medication abortion process midway by giving a woman a high dose of the hormone progesterone after she’s taken the first pill but before she’s ingested the second — at that point, an abortion hasn’t yet occurred.
Heartbeat International told CBS MoneyWatch the treatment has “saved” 5,000 babies of women who have sought to halt a medication abortion. It added that the cost of the treatment varies on the dose of progesterone used in the process. The group also vowed to “continue to offer support to those who seek it.”
Is abortion pill reversal safe?
Heartbeat’s website about abortion pill reversal claims the process is effective and says it “increases the chances” of a pregnancy continuing. The site also contends that the treatment can save 64% to 68% of pregnancies, although it only cites “initial studies” for the statistic, without identifying the specific research.
According to James’ suit, however, “no competent and reliable scientific evidence exists to substantiate these claimed success rates.” As a result, the complaint alleges such claims may mislead consumers about the treatment’s efficacy.
The lawsuit also claims that Heartbeat and other anti-abortion clinics describe the process as “proven safe and essentially risk-free,” while in fact the treatment hasn’t been tested in any reputable medical study.
In a response to CBS MoneyWatch, Heartbeat said the treatment “stands as a safe and effective option for women who change their minds immediately after taking the abortion pill, mifepristone.” The group noted that progesterone, the hormone used in the treatment, is an FDA-approved medication that has been used “for decades to prevent miscarriage and preterm birth.”
Heartbeat added that its assertions are supported by “both scientific evidence and the lived experience of women who are holding their babies in their arms today after starting a chemical abortion and experiencing a successful reversal.”
However, medical experts agree that abortion pill reversal hasn’t been proven safe, as the only double-blind, placebo-controlled, randomized clinical trial for the treatment had to be stopped after some women experienced “life-threatening complications like hemorrhage,” said Dr. Stacy Sun, an obstetrician and gynecologist and a fellow with Physicians for Reproductive Health, a group of doctors that advocates for reproductive health.
“This dangerous, non-evidence based regimen is used to prey on people making thoughtful decisions about their bodies, families and futures,” Dr. Sun told CBS MoneyWatch. “It is infantilizing and manipulative.”
In its countersuit, Heartbeat said that its claims the treatment has been effective in 64% to 68% of pregnancies are based on a 2018 study by Dr. George Delgado, a family physician who created the abortion pill reversal treatment. But James’ lawsuit notes that the study was based on tracking women who called the abortion pill reversal hotline and wasn’t a randomized, placebo-controlled trial.
Meanwhile, some states — primarily those that have restricted abortion access after Roe v. Wade was overturned — “actually require misinformation about medication abortion” to be provided to women who are seeking abortions, the Guttmacher Institute said in an email.
For instance, Nebraska requires that such patients be told: “If you change your mind and want to continue your pregnancy after taking mifepristone, it may not be too late.” They are then directed to Heartbeat’s abortion pill reversal hotline.
“Note that this is misinformation about medication abortion generally, and some of these states put it out in their materials” even if it isn’t required by statutes or regulations, the Guttmacher Institute said.
Heartbeat in February filed a suit seeking to throw out the California case, arguing that the organization is protected by the First Amendment in providing information about abortion pill reversal. The group’s complaint also argues that it is “not appropriate to litigate the merits of scientific studies in the judicial system.”
Heartbeat, which doesn’t itself operate any clinics but provides support to about 3,000 anti-abortion rights centers, said it “receives no kickback or other payment for referring a woman to a physician” in the network of clinics offering abortion pill reversal, according to its suit.
“With this lawsuit, [Attorney General] James is protecting Big Abortion in New York while denying women in her state the right to continue their own pregnancies,” said Jor-El Godsey, president of Heartbeat International, said in the statement sent to CBS MoneyWatch.
CBS News
Trump makes more Cabinet picks but some top economic posts remain unfilled

Watch CBS News
Be the first to know
Get browser notifications for breaking news, live events, and exclusive reporting.
CBS News
Open: This is “Face the Nation with Margaret Brennan,” Nov. 24, 2024

Watch CBS News
Be the first to know
Get browser notifications for breaking news, live events, and exclusive reporting.
CBS News
Popular gluten free tortilla strips recalled over possible contamination with wheat

A food company known for popular grocery store condiments has recalled a package of tortilla strips that may be contaminated with wheat, the U.S. Food and Drug Administration said Friday. The product is meant to be gluten-free.
Sugar Foods, a manufacturing and distribution corporation focused mainly on various toppings, artificial sweeteners and snacks, issued the recall for the “Santa Fe Style” version of tortilla strips sold by the brand Fresh Gourmet.
“People who have a wheat allergy or severe sensitivity to wheat run the risk of serious or life-threatening allergic reaction if they consume the product,” said Sugar Foods in an announcement posted by the FDA.
Packages of these tortilla strips with an expiration date as late as June 20, 2025, could contain undeclared wheat, meaning the allergen is not listed as an ingredient on the label. The Fresh Gourmet product is marketed as gluten-free.
Sugar Foods said a customer informed the company on Nov. 19 that packages of the tortilla strips actually contained crispy onions, another Fresh Gourmet product normally sold in a similar container. The brand’s crispy onion product does contain wheat, and that allergen is noted on the label.
U.S. Food and Drug Administration
No illnesses tied to the packaging mistake have been reported, according to the announcement from Sugar Foods. However, the company is still recalling the tortilla strips as a precaution. The contamination issue may have affected products distributed between Sept. 30 and Nov. 11 in 22 states: Arizona, California, Colorado, Florida, Georgia, Iowa, Idaho, Illinois, Indiana, Maryland, Maine, Michigan, Minnesota, North Carolina, New Jersey, Ohio, Oregon, Pennsylvania, Texas, Utah, Virginia and Washington.
Sugar Foods has advised anyone with questions about the recall to contact the company’s consumer care department by email or phone.
CBS News reached out to Sugar Foods for more information but did not receive an immediate reply.
This is the latest in a series of food product recalls affected because of contamination issues, although the others involved harmful bacteria. Some recent, high-profile incidents include an E. coli outbreak from organic carrots that killed at least one person in California, and a listeria outbreak that left an infant dead in California and nine people hospitalized across four different states, according to the Center for Disease Control and Prevention. The E. coli outbreak is linked to multiple different food brands while the listeria outbreak stemmed from a line of ready-to-eat meat and poultry products sold by Yu-Shang Foods.