CBS News
From Dr. Oz to heart valves: A tiny device charted a contentious path through the FDA

In 2013, the FDA approved an implantable device to treat leaky heart valves. Among its inventors was Mehmet Oz, the former television personality and former U.S. Senate candidate widely known as “Dr. Oz.”
In online videos, Oz has called the process that brought the MitraClip device to market an example of American medicine firing “on all cylinders,” and he has compared it to “landing a man on the moon.”
MitraClip was designed to spare patients from open-heart surgery by snaking hardware into the heart through a major vein. Its manufacturer, Abbott, said it offered new hope for people severely ill with a condition called mitral regurgitation and too frail to undergo surgery.
Photo illustration of 2019 FDA document
“It changed the face of cardiac medicine,” Oz said in a video.
But since MitraClip won FDA approval, versions of the device have been the subject of thousands of reports to the agency about malfunctions or patient injuries, as well as more than 1,100 reports of patient deaths, FDA records show. Products in the MitraClip line have been the subject of three recalls. A former employee has alleged in a federal lawsuit that Abbott promoted the device through illegal inducements to doctors and hospitals. The case is pending, and Abbott has denied illegally marketing the device. The MitraClip story is, in many ways, a cautionary tale about the science, business and regulation of medical devices.
Manufacturer-sponsored research on the device has long been questioned. In 2013, an outside adviser to the FDA compared some of the data marshaled in support of its approval to “poop.”
The FDA expanded its approval of MitraClip to a wider set of patients in 2019, based on a clinical trial in which Abbott was deeply involved and despite conflicting findings from another study.
In the three recalls, the first of which warned of potentially deadly consequences, neither the manufacturer nor the FDA withdrew inventory from the market. The company told doctors it was OK for them to continue using the recalled products.
In response to questions for this article, both Abbott and the FDA described MitraClip as safe and effective.
“With MitraClip, we’re addressing the needs of people with MR who often have no other options,” Abbott spokesperson Brent Tippen said. “Patients suffering from mitral regurgitation have severely limited quality of life. MitraClip can significantly improve survival, freedom for hospitalization and quality of life via a minimally invasive, now common procedure.”
An FDA spokesperson, Audra Harrison, said patient safety “is the FDA’s highest priority and at the forefront of our work in medical device regulation.”
She said reports to the FDA about malfunctions, injuries and deaths that the device may have caused or contributed to are “consistent” with study results the FDA reviewed for its 2013 and 2019 approvals.
In other words: They were expected.
Inspiration in Italy
When a person has mitral regurgitation, blood flows backward through the mitral valve. Severe cases can lead to heart failure.
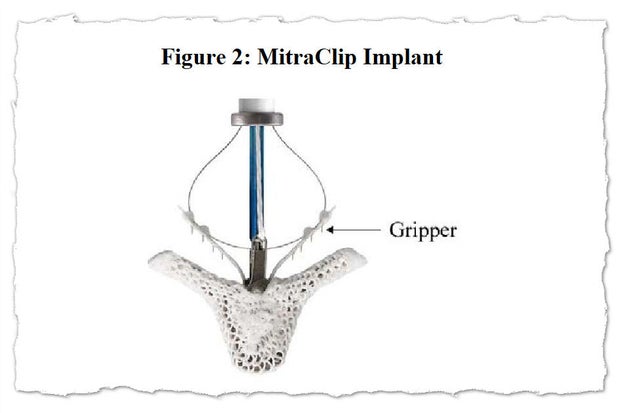
With MitraClip, flaps of the valve — known as “leaflets” — are clipped together at one or more points to achieve a tighter seal when they close. The clips are deployed via a catheter threaded through a major vein, typically from an incision in the groin. The procedure offers an alternative to connecting the patient to a heart-lung machine and repairing or replacing the mitral valve in open-heart surgery.
Oz has said in online videos that he got the idea after hearing a doctor describe a surgical technique for the mitral valve at a conference in Italy. “And on the way home that night, on a plane heading back to Columbia University, where I was on the faculty, I wrote the patent,” he told KFF Health News.
A patent obtained by Columbia in 2001, one of several associated with MitraClip, lists Oz first among the inventors.
Photo by Leigh Vogel/Getty Images for Concordia Summit
But a Silicon Valley-based startup, Evalve, would develop the device. Evalve was later acquired by Abbott for about $400 million.
“I think the engineers and people at Evalve always cringe a little bit when they see Mehmet taking a lot of, you know, basically claiming responsibility for what was a really extraordinary team effort, and he was a small to almost no player in that team,” one of the company’s founders, cardiologist Fred St. Goar, told KFF Health News.
Oz did not respond to a request for comment on that statement.
As of 2019, the MitraClip device cost $30,000 per procedure, according to an article in a medical journal. According to the Abbott website, more than 200,000 people around the world have been treated with MitraClip.
Oz filed a financial disclosure during his unsuccessful run for the U.S. Senate in 2022 that showed him receiving hundreds of thousands of dollars in annual MitraClip royalties.
Abbott recently received FDA approval for TriClip, a variation of the MitraClip system for the heart’s tricuspid valve.
Endorsed “with trepidation”
Before the FDA said yes to MitraClip in 2013, agency staffers pushed back.
Abbott had originally wanted the device approved for “patients with significant mitral regurgitation,” a relatively broad term. After the FDA objected, the company narrowed its proposal to patients at too-high risk for open-heart surgery.
Even then, in an analysis, the FDA identified “fundamental” flaws in Abbott’s data.
One example: The data compared MitraClip patients with patients who underwent open-heart surgery for valve repair — but the comparison might have been biased by differences in the expertise of doctors treating the two groups, the FDA analysis said. While MitraClip was implanted by a highly select, experienced group of interventional cardiologists, many of the doctors doing the open-heart surgeries had performed only a “very low volume” of such operations.
FDA “approval is not appropriate at this time as major questions of safety and effectiveness, as well as the overall benefit-risk profile for this device, remain unanswered,” the FDA said in a review prepared for a March 2013 meeting of a committee of outside advisers to the agency.
Some committee members expressed misgivings. “If your right shoe goes into horse poop and your left shoe goes into dog poop, it’s still poop,” cardiothoracic surgeon Craig Selzman said, according to a transcript.
The committee voted 5-4 against MitraClip on the question of whether it proved effective. But members voted 8-0 that they considered the device safe and 5-3 that the benefits of the device outweighed its risks.
Selzman voted yes on the last question “with trepidation,” he said at the time.
In October 2013, the FDA approved the MitraClip Clip Delivery System for a narrower group of patients: those with a particular type of mitral regurgitation who were considered a surgery risk.
“The reality is, there is no perfect procedure,” said Jason Rogers, an interventional cardiologist and University of California-Davis professor who is an Abbott consultant. The company referred KFF Health News to Rogers as an authority on MitraClip. He called MitraClip “extremely safe” and said some patients treated with it are “on death’s door to begin with.”
“At least you’re trying to do something for them,” he said.
Conflicting studies
In 2019, the FDA expanded its approval of MitraClip to a wider set of patients.
The agency based that decision on a clinical trial in the United States and Canada that Abbott not only sponsored but also helped design and manage. It participated in site selection and data analysis, according to a September 2018 New England Journal of Medicine paper reporting the trial results. Some of the authors received consulting fees from Abbott, the paper disclosed.
A separate study in France reached a different conclusion. It found that, for some patients who fit the expanded profile, the device did not significantly reduce deaths or hospitalizations for heart failure over a year.
The French study, which appeared in the New England Journal of Medicine in August 2018, was funded by the government of France and Abbott. As with the North American study, some of the researchers disclosed they had received money from Abbott. However, the write-up in the journal said Abbott played no role in the design of the French trial, the selection of sites, or in data analysis.
Gregg Stone, one of the leaders of the North American study, said there were differences between patients enrolled in the two studies and how they were medicated. In addition, outcomes were better in the North American study in part because doctors in the U.S. and Canada had more MitraClip experience than their counterparts in France, Stone said.
Stone, a clinical trial specialist with a background in interventional cardiology, acknowledged skepticism toward studies sponsored by manufacturers.
“There are some people who say, ‘Oh, well, you know, these results may have been manipulated,'” he said. “But I can guarantee you that’s not the truth.”
“Nationwide scheme”
A former Abbott employee alleges in a lawsuit that after MitraClip won approval, the company promoted the device to doctors and hospitals using inducements such as free marketing support, the chance to participate in Abbott clinical trials and payments for participating in “sham speaker programs.”
The former employee alleges that she was instructed to tell referring physicians that if they observed mitral regurgitation in their patients to “just send it” for a MitraClip procedure because “everything can be clipped.” She also alleges that, using a script, she was told to promote the device to hospital administrators based on financial advantages such as “growth opportunities through profitable procedures, ancillary tests, and referral streams.”
The inducements were part of a “nationwide scheme” of illegal kickbacks that defrauded government health insurance programs including Medicare and Medicaid, the lawsuit claims.
The company denied doing anything illegal and said in a court filing that “to help its groundbreaking therapy reach patients, Abbott needed to educate cardiologists and other healthcare providers.”
Those efforts are “not only routine, they are laudable — as physicians cannot use, or refer a patient to another doctor who can use, a device that they do not understand or in some cases even know about,” the company said in the filing.
Under federal law, the person who filed the suit can receive a share of any money the government recoups from Abbott. The suit was filed by a company associated with a former employee in Abbott’s Structural Heart Division, Lisa Knott. An attorney for the company declined to comment and said Knott had no comment.
Reports to the FDA
As doctors started using MitraClip, the FDA began receiving reports about malfunctions and cases in which the product might have caused or contributed to a death or an injury.
According to some reports, clips detached from valve flaps. Flaps became damaged. Procedures were aborted. Mitral leakage worsened. Doctors struggled to control the device. Clips became “entangled in chordae” — cord-like structures also known as heartstrings that connect the valve flaps to the heart muscle. Patients treated with MitraClip underwent corrective operations.
As of March 2024, the FDA had received more than 17,000 reports documenting more than 22,000 “events” involving mitral valve repair devices, FDA data shows. All but about 200 of those reports mention one iteration of MitraClip or another, a KFF Health News review of FDA data found.
Almost all the reports came from Abbott. The FDA requires manufacturers to submit reports when they learn of mishaps potentially related to their devices.
The reports are not proof that devices caused problems, and the same event might be reported multiple times. Other events may go unreported.
Despite the reports’ limitations, the FDA provides an analysis of them for the public on its website.
MitraClip’s risks weren’t a surprise.
Like the rapid-fire fine print in television ads for prescription drugs, the original product label for the device listed more than 60 types of potential complications.
Indeed, during clinical research on the device, about 6% of patients implanted with MitraClip died within 30 days, according to the label. Almost 1 in 4 — 23.6% – were dead within a year.
The FDA spokesperson, Harrison, pointed to a study originally published in 2021 in The Annals of Thoracic Surgery, based on a central registry of mitral valve procedures, that found lower rates of death after MitraClip went on the market.
“These data confirmed that the MitraClip device remains safe and effective in the real-world setting,” Harrison said.
But the study’s authors, several of whom disclosed financial or other connections to Abbott, said data was missing for more than a quarter of patients one year after the procedure.
A major measure of success would be the proportion of MitraClip patients who are alive “with an acceptable quality of life” a year after undergoing the procedure, the study said. Because such information was available for fewer than half of the living patients, “we have omitted those outcomes from this report,” the authors wrote.
If you’ve had an experience with MitraClip or another medical device and would like to tell KFF Health News about it, click here to share your story with us.
KFF Health News audience engagement producer Tarena Lofton contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling and journalism.
CBS News
12/16: The Daily Report – CBS News

Watch CBS News
Be the first to know
Get browser notifications for breaking news, live events, and exclusive reporting.
CBS News
Takeaways from Trump’s first post-election news conference

Watch CBS News
Be the first to know
Get browser notifications for breaking news, live events, and exclusive reporting.
CBS News
The health stories that shaped 2024

Watch CBS News
Be the first to know
Get browser notifications for breaking news, live events, and exclusive reporting.